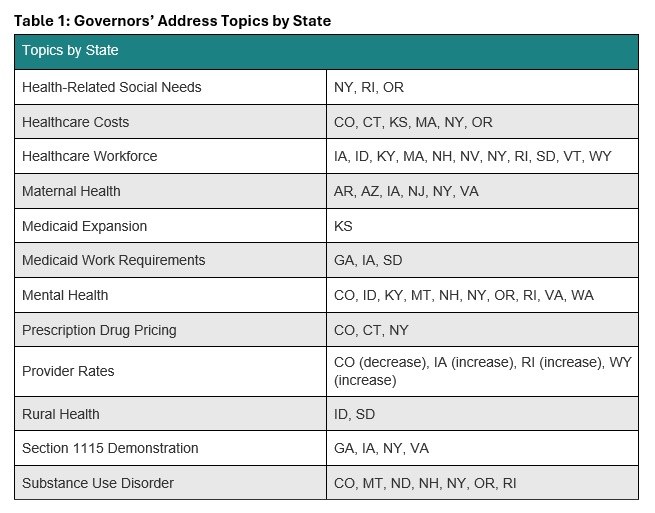
As policymakers engage the state budget process, Medicaid continues to play a critical role. This role is programmatic, serving as the source of coverage for 1 in 4 Ohioans, including as the finance mechanism for half of all births in the state and the primary source of coverage for the elderly and disabled. However, a number of proposals are currently being discussed by the House, including changing how poverty programs are adjusted for inflation, reversing some Medicaid payment expansions, lowering the minimum federal funding rate for Medicaid, making the federal funding rate the same for all Medicaid expansion populations, limiting taxes on Medicaid providers, capping the amount spent per Medicaid enrollee, standardizing how administrative costs are matched, and other unspecified changes to Medicaid funding through Medicaid match. But these proposals can’t be viewed in isolation because the program is deeply intertwined with Ohio’s ability to have a balanced budget, serving a role in reducing direct state spending by enabling the draw down of federal dollars through “FMAP.” But what is FMAP and what happens if Congress fundamentally changes how it’s calculated?
FMAP
The Federal Medical Assistance Percentage (FMAP) is a critical component of Medicaid funding, ensuring that states receive federal support to provide healthcare services to low-income individuals. FMAP is calculated based on a state’s per capita income relative to the national average. States with lower per capita incomes receive a higher FMAP, meaning the federal government covers a larger share of Medicaid costs, while states with higher per capita incomes receive a lower FMAP. The FMAP formula ensures that states with greater financial need, like Ohio, receive more federal assistance. For Federal Fiscal Year (FFY) 2025, Ohio’s FMAP is 64.6%, which means that for every dollar Ohio spends on most Medicaid services, 64.6 cents comes from the federal government. Even then, much of the state share is financed through fees on entities like hospitals, nursing facilities and health insurance companies.
Contextually, Ohio is a “recipient state,” indicating it receives more in federal tax revenue than it collects to finance the program. And, as was noted in the initial testimony offered in the Ohio House, Ohio continues to lag other states in terms of economic growth and has an aging population. As such, the availability and predictability of federal funding is a critical input in future years, particularly in long term care where most of the expense will continue to increase. With Congress deliberating all of these proposals, what could the impact be in Ohio? To illustrate, it may be good to focus on one area: the elimination of enhanced federal funding for those covered by the Medicaid expansion.
Impacts
There has been some discussion during testimony that if the FMAP rate for the expansion population were to change, it could trigger an automatic end to the expansion itself. Importantly, there would be a disproportionate impact in Ohio’s rural counties where expansion coverage rates are higher. In fact, as of December 2024, 362,829 individuals in rural Ohio counties received their coverage through expansion, alone. These individuals, in addition to the 1.1 million others in these Ohio counties, rely on Medicaid for essential healthcare services, including addiction treatment.
In states with expansion, coverage for individuals with SUD has doubled highlighting the importance of maintaining robust funding for these programs. Expansion has also been the primary source of funding for addiction treatment in the state, with Medicaid covering half of all buprenorphine treatments. If expansion were eliminated due to the change in FMAP, the consequences for treatment may mean either a greater obligation on the state to finance those services directly, or, Ohio may exacerbate the opioid use disorder crisis, putting additional strain on our healthcare system, particularly for behavioral health providers.
Conclusion
As we consider the future of Ohio’s Medicaid expansion, it’s essential to recognize the critical role that FMAP plays in sustaining our healthcare system and supporting our state’s economy. Any changes to the FMAP rate must be carefully evaluated to ensure that we do not undermine the progress we have made in expanding access to care and addressing the opioid crisis.
Beyond the immediate impact on healthcare services, changes to FMAP could also have broader economic implications for Ohio. Medicaid represents about 4% of the state’s GDP, playing a vital role in supporting jobs and economic activity. If just 1% of that GDP were suddenly eliminated due to a cut in federal or state funding, the consequences could be severe. A sudden reduction in Medicaid funding could lead to job losses in the healthcare sector, reduced economic activity, reduced labor force participation, and increased financial strain on state and local governments. The ripple effects would be felt across the economy, impacting not only healthcare providers but also businesses and communities that rely on the stability and support provided by Medicaid as well as the budgetary stability it provides to Ohio’s process.