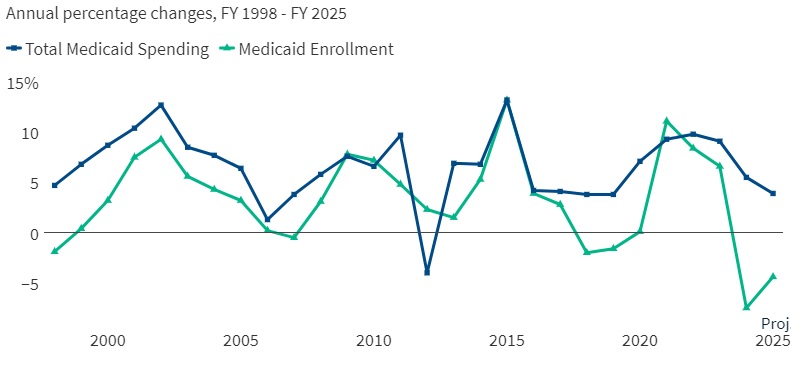
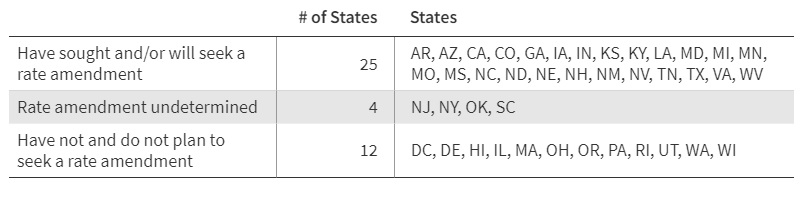
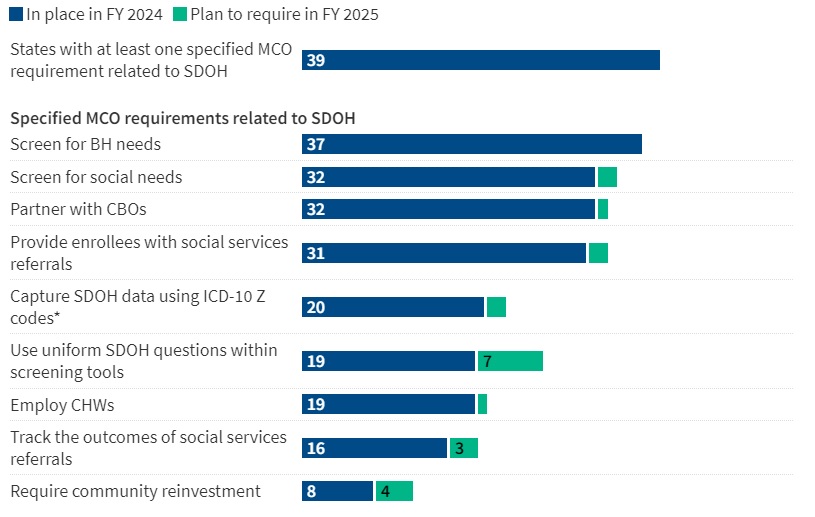
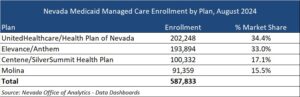
This week’s In Focus section reviews new state initiatives to cover traditional healing services through Medicaid for American Indian/Alaska Native (AI/AN) individuals and communities.
Overview
In October 2024, The Centers for Medicare & Medicaid Services (CMS) approved Medicaid Section 1115 demonstration amendments for Arizona, California, New Mexico, and Oregon, allowing Medicaid and Children’s Health Insurance Program (CHIP) coverage of traditional healing services delivered at or through Indian Health Service facilities, Tribal facilities, and urban Indian organizations (I/T/U facilities).
This demonstration approval enables state Medicaid agencies to acknowledge traditional healthcare practices as important components of the wellness continuum of care for Native American populations. Medicaid funding will help strengthen and expand access to these services and support integration of these services into primary care, substance use disorder (SUD) treatment, and other behavioral health care in a way that I/T/U providers have designed and developed to meet the unique needs of their community.
Demonstrations for Arizona and Oregon are approved through September 30, 2027, New Mexico’s demonstration is authorized through December 31, 2029, and California’s through December 31, 2026.
Traditional Health Services: Providing Culturally Relevant Care
AI/AN populations generally experience worse health disparities compared with non-AI/AN populations, particularly in terms of obesity, diabetes, tobacco addiction, and cancer. AI/AN populations also face higher rates of mental health disorders, SUDs, and suicide.
Using Transformed Medicaid Statistical Information System (T-MSIS) claims and demographics data, Health Management Associates, Inc. (HMA), staff assessed the incidence of specific chronic diseases in the Native American and non-Native American population in the four states approved to cover traditional healing services through their Medicaid program. For example, in these states, the prevalence of diabetes in AI/AN populations ranged from 27 percent to 87 percent higher than among non-AI/AN groups. Figure 1 shows the percentage of three chronic conditions among these groups in the four states.
Figure 1. Percentage of AI/AN vs. Non-AI/AN Medicaid Beneficiaries Living with Chronic Conditions in AZ, CA, NM, and OR (2022)
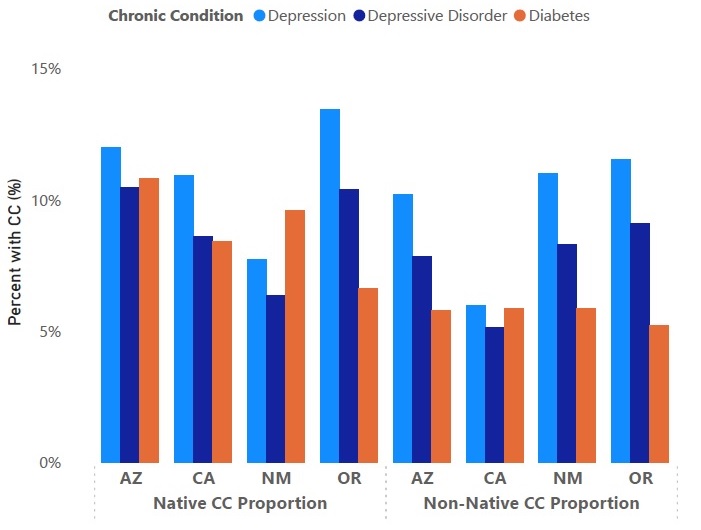
The demonstration approval is expected to improve access to culturally appropriate healthcare to address these disparities in chronic conditions for Tribal communities. Traditional healthcare practices vary widely across the 574 federally recognized Tribes in the United States, and many see traditional healthcare practices as a fundamental element of well-being that can help patients with specific physical and behavioral health conditions. For example, commonly offered traditional practices in Native American communities include talking circles, sweat lodges, and smudging. Studies show that incorporating traditional healthcare practices may improve mental health symptoms, outcomes, and quality of life, including among individuals with SUD.
Considerations for Key Partners
AZ, CA, NM, and OR are the first states to receive federal approval and will lay the groundwork for integrating time-honored healing practices into their Medicaid systems. They also could serve as a model for other states that choose to pursue this demonstration. I/T/Us were integral to shaping the demonstration design and are poised to continue shaping the program details and implementation of traditional approaches to care into their Medicaid systems.
HMA experts identified some key considerations for partners, such as states and Medicaid managed care organizations (MCOs), to follow as these services are incorporated into Medicaid:
- Collaborate with I/T/U facilities and communities. Traditional healing practices are sacred and ceremonial, so flexibility will be essential in determining how Medicaid funding can be best allocated to support providers who offer traditional practices. Communities will be critical in helping identify the specific traditional healing practices that are needed.
- Support operational changes needed in I/T/U facilities. Compliant and efficient billing practices will be essential to the success of the demonstrations. States can support I/T/U facilities to develop necessary trainings, workflows, and administrative processes. For example, the provider qualification criteria and implementation is central to meeting federal compliance and reporting requirements. Facilities also will need to meet Medicaid billing requirements to collect 100 percent of the federal medical assistance percentage (FMAP).
- Partner with I/T/U facilities. To facilitate proper care coordination, states, health plans, and non-I/T/U providers should partner with I/T/U facilities to ensure patients experience the best health outcomes.
Connect With Us
HMA has learned the value and importance of working with Native American and Alaska Native populations and respecting their unique approaches to improving healthcare. HMA has expertise on healthcare issues that uniquely affect AI/AN populations and is experienced in addressing these challenges through AI/AN leadership and engagement that is culturally sensitive and respectful. Our experience working directly with Tribes encompasses extensive and applicable knowledge of healthcare operations in rural and urban settings to support infrastructure needs, including data management, IT, staffing, policies and procedures, training, and eligibility and enrollment processes.
Contact our featured expert below to learn more about HMA’s work to support Native American and Alaska Native communities.