This week, our In Focus section examines how the federal government implemented changes to the Medicare program in response to COVID-19. As the COVID-19 pandemic began in the United States, Congress and the Administration responded with a series of legislative, regulatory, and sub-regulatory changes to the Medicare program that were designed to provide relief from certain Medicare rules to assist health care providers, Medicare Advantage organizations, and Part D plans in responding to the pandemic. Some of these changes waived conditions of Medicare participation to enable patients to be treated in alternative care settings. Others permitted physicians and other providers to receive Medicare reimbursements for telemedicine services.
Between January 1 and July 24, 2020, Congress and the administration modified more than 200 Medicare policies. The Centers for Medicare & Medicaid Services (CMS) issued sub-regulatory guidance on a near weekly basis during this time to provide flexibility to providers and Medicare plans.
Health Management Associates (HMA) cataloged the array of COVID-19-related regulatory changes during this time period and categorized them according to their characteristics, including types of providers and plans affected, effective date, and expected duration. This information is available in the companion policy tracker available here.
Our analysis found that actions taken by Congress and the Administration in response to the COVID-19 pandemic affected virtually all types of health care providers and health plans that participate in the Medicare program. We depict characteristics of COVID 19-related regulatory changes in Exhibit 1 and summarize findings in the paragraphs that follow.
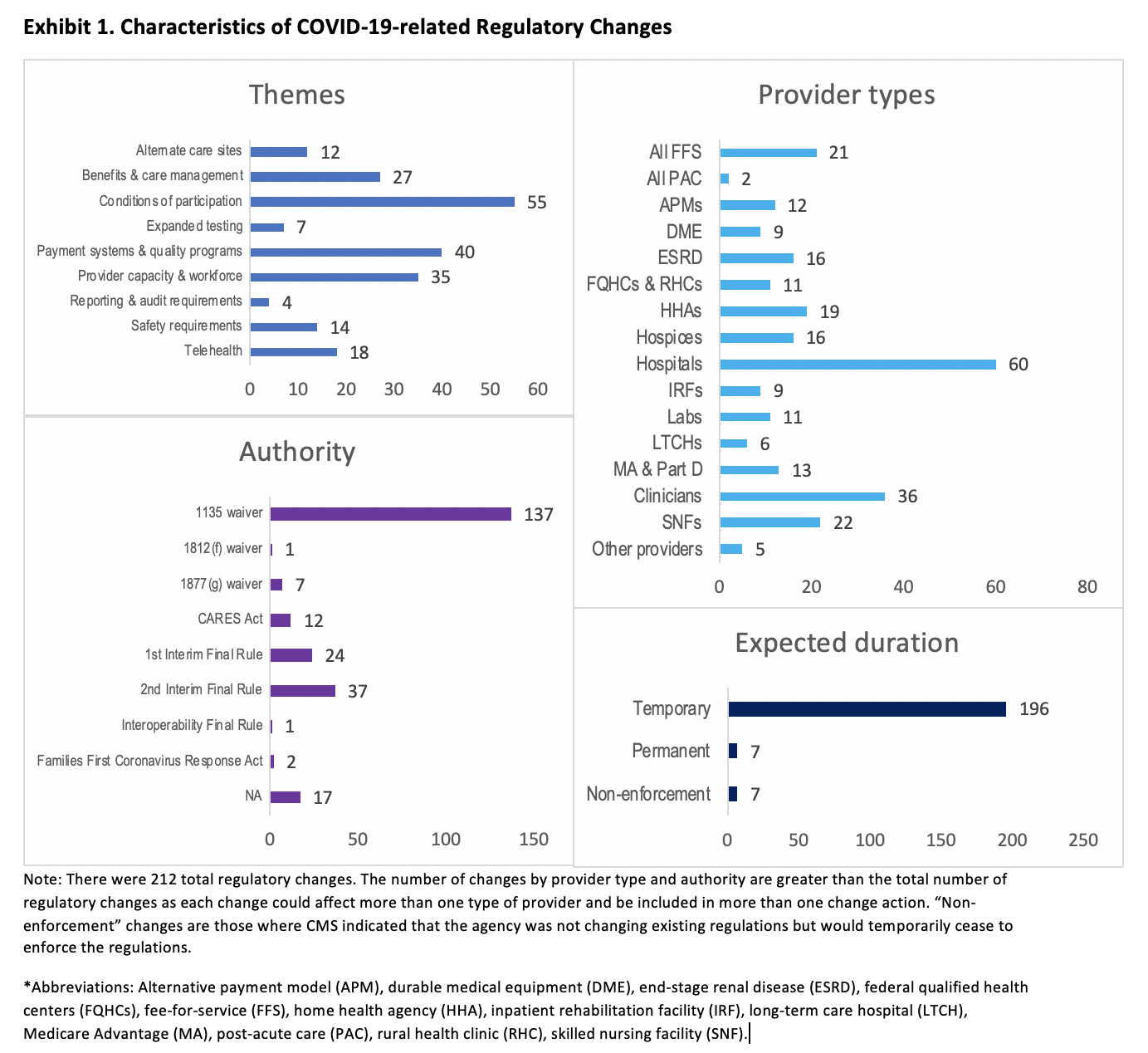
Themes. We organized the policies into nine themes. According to our categorization, the actions of Congress and the Administration primarily addressed conditions of participation requirements for Medicare providers (55). The next most common themes were payment systems and quality programs (40) and provider capacity and workforce (35).
Provider Types. To date, efforts have focused on hospital providers, accounting for 60 of the 212 policy actions.
Authority. Most policies (145) were implemented through the Depart of Health and Human Services’ various waiver authorities (Exhibit 1). CMS implemented 61 changes through two interim final rules with comment periods.
Expected Duration. Nearly all the policy actions (203) are currently expected to be temporary, absent further Congressional or administration action. Seven of the actions were not direct changes to or waivers of regulations; instead, CMS indicated that it would not enforce the existing regulations for a limited time. While most COVID-19-related regulatory changes were declared to be temporary when they were announced, the administration has indicated plans to make some permanent. Changes that have proven popular with providers and patients are likely candidates, such as expanded reimbursement for telemedicine services.
The full issue brief and companion policy tracker can be found here. For more information on the changes discussed here or other Medicare and COVID-19 policy questions, please contact Jennifer Podulka or Jonathan Blum.