This week, our In Focus, written by HMA Principal Anne Winter and Senior Consultant Aimee Lashbrook, examines American Patients First: The Trump Administration Blueprint to Lower Drug Prices and Reduce Out-of-Pocket Costs, released May 11, 2018. Over time, the pharmaceutical supply chain has become a complex ecosystem, responding to the ever-changing dynamics of new drug products, pricing strategies, health care reform, benefit design, and the regulatory environment making it, arguably, the most complicated in health care. Due to this complexity, solutions to equitably control drug pricing will take a multiprong approach that includes regulatory redesign.
The Blueprint identifies several challenges to addressing drug pricing:
- A business model born on the complexity of supply chain dynamics
- Loss of patent exclusivity and increase in generic alternatives
- Impact of the Affordable Care Act (taxes, rebates)
- Expansion of the 340B drug discount program
- Expansion of international price controls
- Lack of negotiation tools for government programs
- Changes in insurance benefit design that shifts costs to consumers
- Growth in high-cost drugs
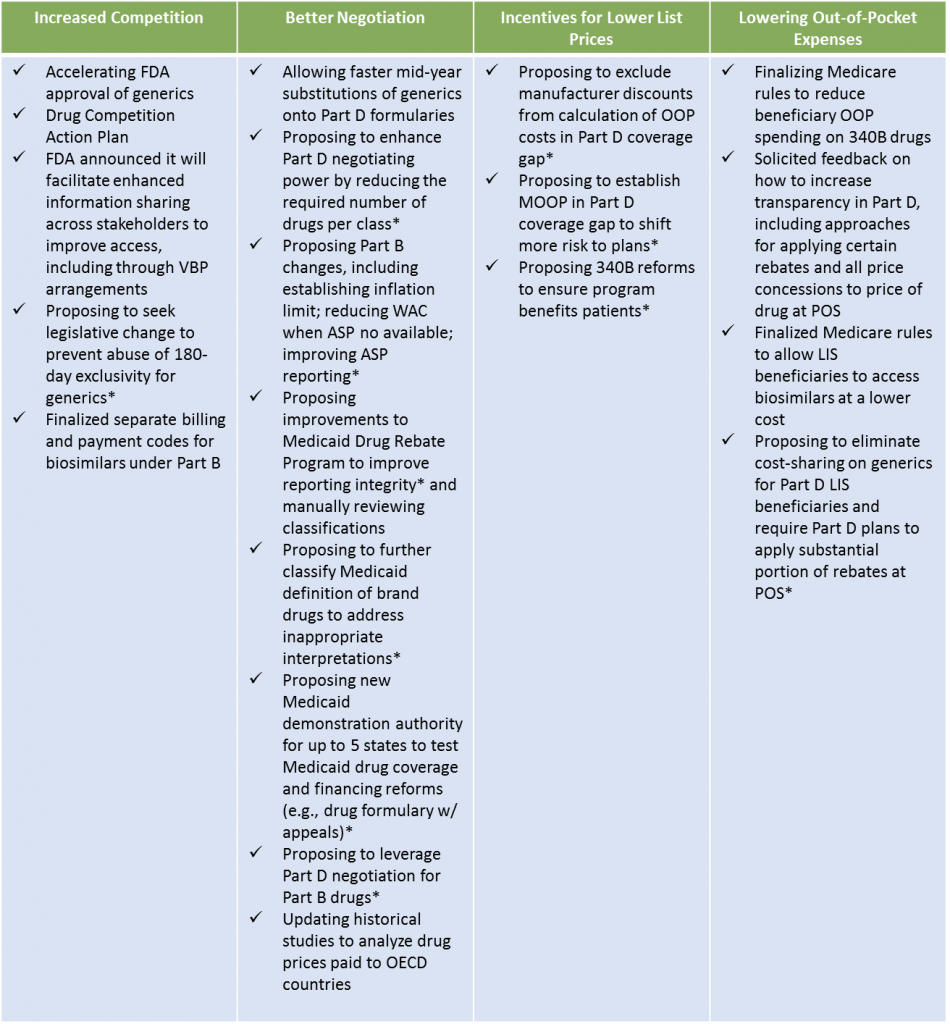
The Blueprint offers multiple strategies to address these challenges:
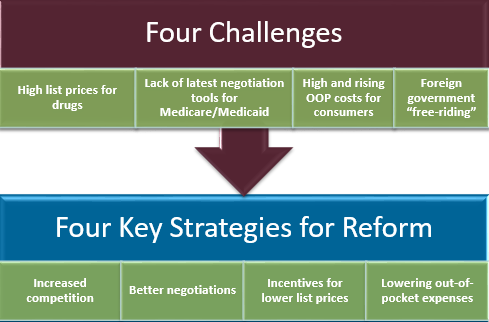
The multiple strategies outlined in the Blueprint are categorized by whether the U.S. Department of Health and Human Services (HHS) believes they can be addressed now, in the short term, or whether they require additional research and stakeholder input. HHS believes it can take many actions now within the current regulatory environment. These actions, which may only require a “stroke of a pen”, and more long-term solutions that will require additional stakeholder feedback, are outlined in the tables below. HHS is requesting public comment on many of these longer-term solutions and interested parties should participate in this stakeholder feedback process.
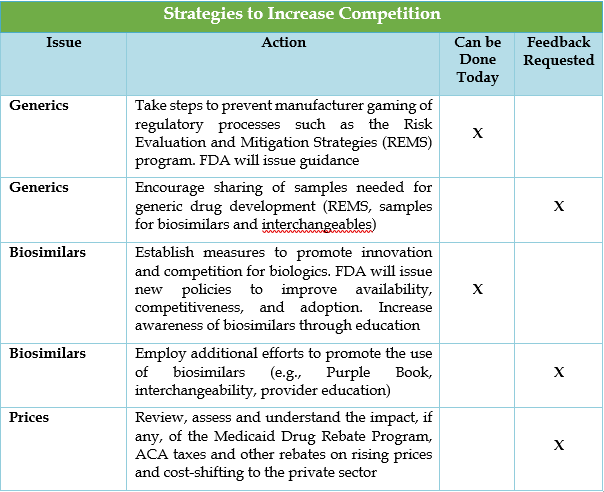
The majority of the strategies to increase competition involve bringing more therapeutic alternatives to single source brand drugs to market. Increased competition is accomplished through improving pathways for generic drug and biosimilar development and prescribing.
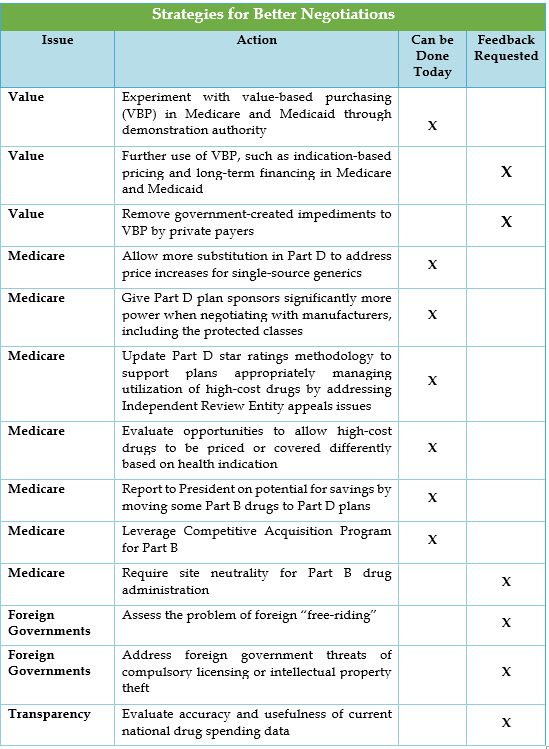
Strategies for better negotiations focus on value-based purchasing of drugs, Medicare Part B and D, and foreign government actions. Medicare reforms include allowing Part D plans to negotiate tighter formularies and administering drugs normally paid through the Part B benefit.
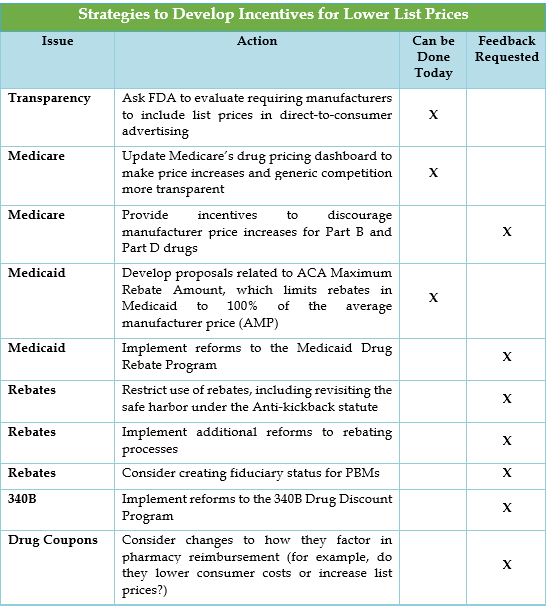
Strategies to incentivize lower list prices focus on government programs, pharmacy benefit manager (PBM) rebate strategies, the 340B drug discount program, and drug coupons. Rebates have a particular focus due to the dynamic between the list price of drugs and the net price of drugs post-rebate.
All the strategies to reduce out-of-pocket costs are Medicare-driven and focused on providing Medicare beneficiaries with information on drug costs and lower cost alternatives.
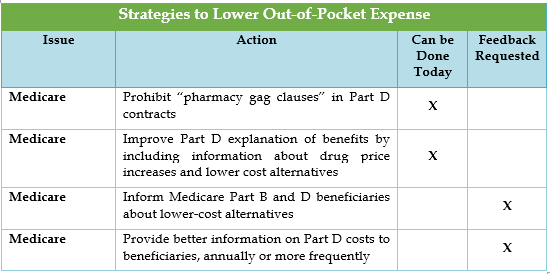
HHS is already taking action on policies outlined in the Blueprint, such as issuing a Health Plan Management System (HPMS) memorandum prohibiting pharmacy gag clauses in Part D contracts, releasing the names of manufacturers who have had complaints filed against them regarding sample availability, and publishing an updated Medicare drug pricing dashboard. These actions are in addition to policies already being pursued by HHS prior to releasing the Blueprint, which are identified in the table below.[1]
For additional information, please contact HMA Principal Anne Winter.
[1] *Proposed in the President’s 2019 budget.
