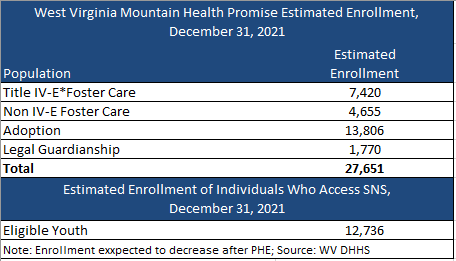
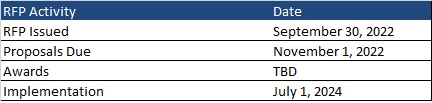
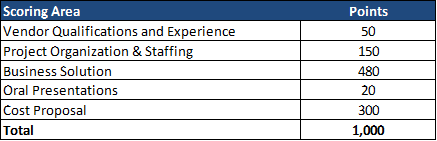
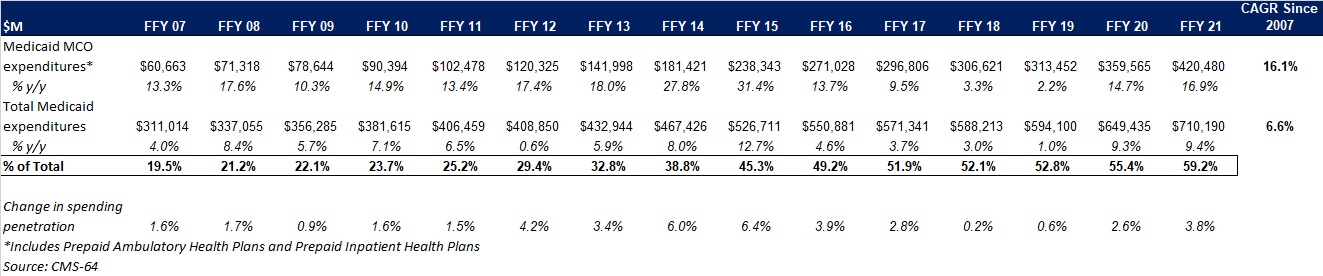
Health Management Associates (HMA) has a rich history of serving the healthcare infrastructure needs of Native American and Alaska Native communities – through healthcare IT support, clinical governance for change management, culturally competent stakeholder engagement, and revenue cycle management that implements an approach that is tailored to the provider and payer entities that deliver care in Native American and Alaska Native communities.
American Indian and Alaska Native (AI/AN) people experience disproportionately poorer health status compared to Americans as a whole. AI/ANs born today have a life expectancy that is 5.5 years less than the national average for all races. HMA has expertise with healthcare issues that uniquely impact American Indian and Alaska Native populations and is experienced in addressing these issues through American Indian and Alaska Native leadership engagement that is culturally sensitive and respectful.
Case Studies
Examples of HMA’s work with tribal health providers include:
Skokomish Tribal Health Center: Policy, Programs, Operations, And Training
HMA provided training and technical assistance to Skokomish Indian Tribal Health Center in Skokomish, Washington. This support includes the provision of clinical oversight services by one of HMA’s clinicians as Interim Medical Director.
HMA: (1) assessed the Tribal Health Center’s billing practices and assisted in the development of a procurement and contract with a new third-party billing vendor; (2) developed a screening and intervention program for medications for addiction treatment (MAT) of opioid and alcohol use disorders, including training for providers and administrative and medical staff; (3) established policies and procedures to support the clinic to move to a primary care medical home model of care, including development of a back office manual and trainings to clinic staff and providers; and (4) provided technical assistance to the Tribal Health Center as it developed telehealth programs for both video visits and remote patient monitoring (RPM), including development of policies and procedures for maintaining operations during emergencies, procedures and workflow approaches for working with IT vendors and potential purchase and implementation of a new electronic health record.
Fort Belknap Tribes: Assessing Behavioral Health Infrastructure
HMA conducted a feasibility assessment to determine how best Fort Belknap Tribal Health Department could take over administration of behavioral health services through a 638 contract with the Indian Health Service (IHS) agency. To do this, HMA conducted a review of select documentation, contracts, and data sets, including clinical and financial data. Through site visits, HMA conducted focus groups and interviews with tribal, IHS, and community stakeholders. HMA also provided research of other tribal behavioral health programs and interviews with tribes successfully delivering comprehensive behavioral health services.
Chickasaw Nation Department of Family Services: Implementing Practice Changes
HMA provided consultation regarding integration of behavioral health into primary care sites, sharing expertise and advice on privacy and confidentiality regulations and integrated care, how to manage during the transition from traditional behavioral health to integrated care, ideas for including medical family therapy more broadly in patient care, and the implementation of patient assessments.
Montana Healthcare Foundation: Identifying and Developing Opportunities with Tribal and Medicaid Leadership:
The Montana Healthcare Foundation (MHCF) convened tribal health care leaders to develop shared priorities to jointly pursue with the Montana Department of Public Health and Human Services (DPHHS) and the Legislature. HMA was engaged by MHCF to help the group assess implementation options related to the Centers for Medicare and Medicaid Services’ (CMS) revised interpretation of the 100 percent federal match for state Medicaid programs for American Indian Medicaid enrollees for services. HMA proposed options for how DPHHS can use the new match funding to support IHS and tribal health facilities as they implement these new processes and support shared priorities. Priorities identified by the tribal health leaders included operational and policy issues such as improving health information technology capacity; identifying opportunities to compact aspects of health care delivery from IHS; and improving clinic operations through business management training.
Montana Office of Public Instruction SAMHSA Tribal Systems of Care Grantee: Systems of Care Evaluation
HMA served as the independent evaluator for Montana Office of Public Instruction (OPI) Substance Use and Mental Health Services Administration (SAMHSA) Tribal Systems of Care Evaluation. The five-year evaluation assesses the impact of High-Fidelity Wraparound services being provided to American Indian students in schools on six tribal reservations throughout the state. Evaluation activities include tracking quantitative data to measure progress toward grant goals and tracking qualitative data to assess impact of wraparound activities through key informant interviews, small group listening sessions, and site visits. Evaluation findings are regularly reported to SAMHSA and presented locally to key stakeholders.
Montana Tribally Operated Substance Use Disorder (SUD) Continuum of Care Concept Brief
HMA worked in coordination with the American Indian Health Leaders (AIHL) workgroup, a group made up of leadership from Montana’s seven tribes representing tribal health departments and urban Indian health centers, to develop a SUD Continuum of Care Concept brief, describing potential approaches for design and financing of a jointly tribally operated SUD treatment facilities. This work was conducted through a contract with Montana Healthcare Foundation.
Montana Tribally Operated Substance Use Disorder Continuum of Care
HMA provides technical assistance and facilitation and consulting expertise to support the development of a statewide joint tribally operated SUD Continuum of Care. HMA facilitated discussions with AIHL and Chemical Dependency Center (CD) directors. HMA provided subject matter expertise on the various design options available based on American Society of Addiction Medicine (ASAM) service needs criteria as well as analysis support. HMA is also providing consulting support on financial and operational planning for the development of new facilities. This work could include methods for short-term and long-term forecasting and scenario modeling, identification, and negotiation of capital and operational financing for construction and start-up phase, and technical assistance on revenue cycle best practices to ensure satisfactory patient experience and sustainable revenue. This work is being conducted through a contract with the MHCF.
Health Policy and Advocacy
HMA consultants have worked with the following organizations associated with American Indian health policy and advocacy issues:
American Indian Health Commission of Washington State
A tribally driven non-profit organization with a mission of improving health outcomes for American Indians and Alaska Natives through a health policy focus at the Washington state level. AIHC works on behalf of the 29 federally recognized Indian tribes and two urban Indian health organizations.
Southcentral Foundation
An Alaska Native-owned, nonprofit health care delivery and advocacy organization serving nearly 65,000 Alaska Native and American Indian people living in Anchorage, Matanuska-Susitna Borough and 55 rural villages. Southcentral Foundation and the Alaska Native Tribal Health Consortium own and manage the Alaska Native Medical Center that serves the entire Alaska Native and American Indian population in the state.
Northwest Portland Area Indian Health Board
Engaged in many areas of Indian health, including legislation, policy analysis, health promotion and disease prevention, as well as data surveillance and research. The Northwest Portland Area Indian Health Board (NPAIHB) is a non-profit tribal advisory organization serving the forty-three federally recognized tribes of Oregon, Washington, and Idaho.
Messengers for Health
A non-profit organization located on the Apsáalooke (Crow) Reservation in Montana whose mission is to improve the health of individuals on the Crow Indian Reservation and outlying areas through community-based projects that empower communities to assess and address their own unique health-related challenges.
For more information about HMA’s Native American and Alaska Native support services, contact [email protected].