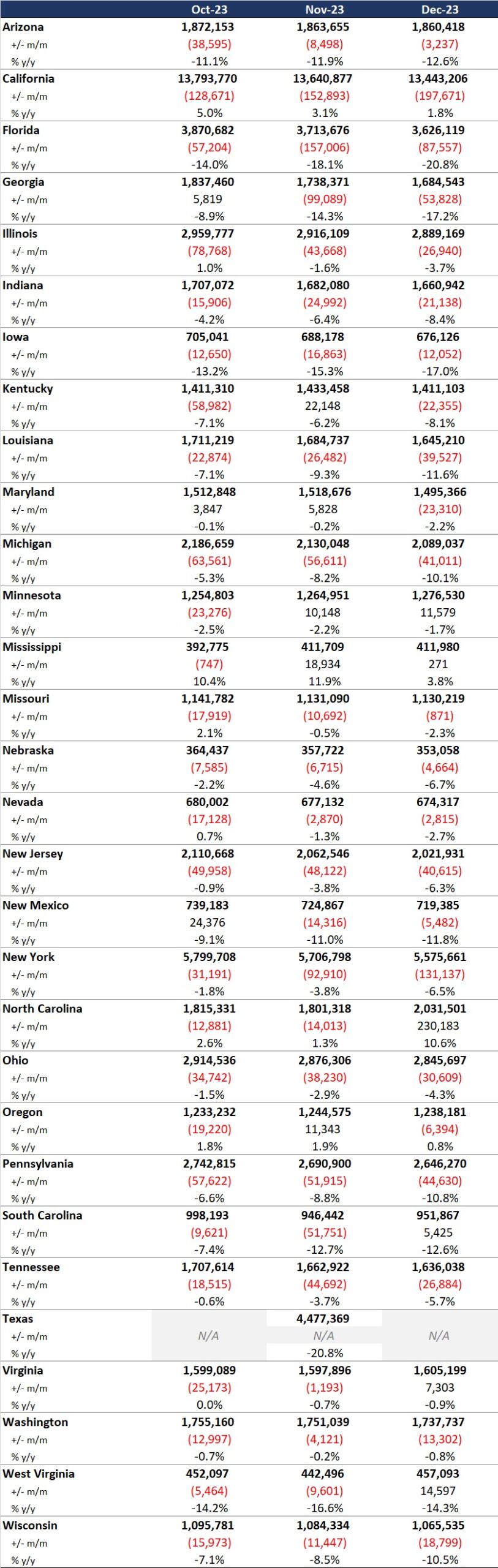
Our second In Focus section reviews the policy changes proposed by the Centers for Medicare & Medicaid Services (CMS) on April 10, 2024, for the Fiscal Year (FY) 2025 Medicare Hospital Inpatient Prospective Payment System (IPPS) and Long-Term Acute Care Hospital (LTCH) Proposed Rule (CMS-1808-P). This year’s IPPS Proposed Rule includes several policy changes that will alter hospital margins and change administrative procedures, beginning as soon as October 1, 2024.
We highlight five proposed policies that are likely to have the greatest impact on Medicare beneficiaries, hospitals and health systems, payors, and manufacturers:
- Annual inpatient market basket update
- New technology add-on payments (NTAP) policy changes
- Transforming Episode Accountability Model (TEAM)
- Hospital wage index and labor market adjustments
- Revision to housing-related diagnosis coding
Stakeholders have until June 10, 2024, to submit comments to CMS on the contents of this regulation and request for information.
Market Basket Update
Proposed rule: Overall CMS’s Medicare 2025 Hospital Inpatient Proposed Rule will increase payments to acute care hospitals by an estimated $3.2 billion in 2024−2025; however, recent trends in economy-wide inflation may alter this estimate by the time the agency releases the final regulation in August 2024.
HMA/Moran analysis: CMS’s 2.6 percent increase is based largely on an estimate of the rate of increase in the cost of a standard basket of hospital goods—the hospital market basket. For beneficiaries, this payment rate increase will lead to a higher standard Medicare inpatient deductible and increase out-of-pocket costs. For hospitals and health systems, payors, and manufacturers the proposed payment increase (2.6%) falls below economywide inflation over the past year (3.5%) and below what Medicare Advantage plans will receive for 2025 (3.7%).1,2 Importantly, based on our expertise with the calculation of the hospital market basket, we anticipate the proposed 2.6 percent increase will increase slightly by the time rates are finalized later this year.
New Technology Add-on Payments (NTAPs)
Proposed Rule: CMS proposes three changes to the NTAP program and discusses NTAP applications for FY 2025:
- CMS proposes to shift the date used to determine whether an otherwise qualifying product is within its newness period. As proposed, if the product’s three-year anniversary occurs after the beginning of the fiscal year on October 1, the product will receive NTAP payments that year.
- CMS proposes to allow products with a hold on their FDA marketing authorization application to be considered eligible for NTAP.
- Beginning with applications approved in the current FY 2025 cycle, the NTAP add-on percentage for gene therapies treating sickle cell disease would increase to 75 percent.
HMA/Moran Analysis: The first two proposed changes are in response to concerns about more restrictive application requirements finalized last year. When CMS shifted the FDA approval deadline to May 1 last year, commenters noted that fewer products would be eligible to receive NTAPs in their third year of the newness period. Allowing all products with a third anniversary that falls within a fiscal year (rather than only those with expirations in the second half of the fiscal year) to receive NTAPs narrowly addresses this concern. More products will qualify for NTAPs during their third year of newness, but that does not necessarily mean that more products will receive three years of NTAPs.
The second proposal tweaks last year’s change requiring a “complete and active” FDA application at the time an NTAP application is submitted to ensure that NTAP applications were far enough along in the FDA review process that information about the product would be available to the public and for CMS staff review. CMS proposal acknowledges that the original bright line rule may have inappropriately excluded potential applicants.
Finally, CMS’s proposal to increase the NTAP percentage for gene therapies treating sickle cell disease aligns with the Cell and Gene Therapy Access Model’s focus on sickle cell therapies. Of note, CMS seeks comment on whether the increased NTAP percentage should be applied only to applicants that have entered value-based purchasing agreements or are “otherwise engaging in behaviors that promote access to these therapies at lower cost.” CMS seems willing to increase NTAP payments in limited situations to boost selected policy goals, but the proposals in this regulation do not represent widespread NTAP payment increases.
Transforming Episode Accountability Model (TEAM)
Proposed Rule: CMS proposes to establish a new mandatory episode-based CMS Innovation Center model, Transforming Episode Accountability Model (TEAM). In the TEAM model, selected acute care hospitals would coordinate care for people with traditional Medicare who undergo one of the five specified surgical procedures:
- Lower extremity joint replacement
- Surgical hip femur fracture treatment
- Spinal fusion
- Coronary artery bypass graft
- Major bowel procedure
Hospitals in the model will assume responsibility for the cost and quality of care from surgery through the first 30 days after the Medicare beneficiary leaves the hospital. Hospitals also must refer patients to primary care services to support optimal long-term health outcomes.
In a first of its kind program, CMS has created a voluntary decarbonization and resilience initiative through which participating hospitals can report metrics related to greenhouse gas emissions to CMS. CMS will provide individualized feedback reports and public recognition of participation and potential performance in the initiative.
HMA/Moran Analysis: The critical aspect of the TEAM model that stakeholders need to understand is that it will be mandatory. TEAM will begin in 2026 and continue for five years. The TEAM model builds on and combines previous models such as the Bundled Payment for Care Improvement (BPCI) model and the Comprehensive Care for Joint Replacement (CJR) model. Hospitals will be required to report various quality measures, and payment will be based on spending targets and include retroactive reconciliation. TEAM also seeks to integrate specialty and primary care. The model complements existing accountable care organization (ACO) models such as ACO REACH or the Medicare Shared Savings Program as beneficiaries would be able to be assigned to both TEAM and ACO programs.
Hospital Wage Index Adjustments and Labor Market Changes:
Proposed Rule: CMS proposes two wage index policies for FY 2025. First, CMS proposes to extend the temporary policy finalized in the FY 2020 IPPS/LTCH PPS final rule for three additional years to address wage index disparities affecting low-wage index hospitals, which includes many rural hospitals. Second, as required by law, CMS proposes to revise the labor market areas used for the wage index based on the most recent core-based statistical area delineations issued by the Office of Management and Budget (OMB) based on 2020 Census data.
HMA/Moran analysis: The two wage index policies that CMS proposes for FY 2025 will have important positive and potentially negative consequences for hospital payment. The policy to extend the low-wage index policy for three additional years will allow many hospitals with low wage indexes to increase their wage index and their payment rates across all MS-DRGs. This policy will bring millions of additional dollars to rural hospitals in FY 2025.
The second policy is a statutorily required update to the labor markets used to establish CMS’s hospital wage indexes. CMS will redefine 53 counties from urban to rural and 54 counties from rural to urban, which will disrupt various hospital payment policies for hospitals in the affected counties. The overall impact of both proposed geographic policy changes for FY 2025 will be to increase inpatient payment rates for rural hospitals.
Revision to Housing-Related Diagnosis Coding
Proposed Rule: CMS proposes to change the severity designation of the seven ICD-10-CM diagnosis codes that describe inadequate housing and housing instability from non-complication or comorbidity (non-CC) to complication or comorbidity (CC).
HMA/Moran Analysis: In proposing this change, CMS is building on its previous policy of including diagnosis codes for describing when a beneficiary is homeless (e.g., unspecified, sheltered, unsheltered). Importantly, this new policy proposal will enable hospitals to be paid higher inpatient payment rates when patients with inadequate or unstable housing are served. Specifically, this proposal would result in cases involving patients to whom these codes apply to be coded in a higher-level MS-DRG within a given family of MS-DRG codes. If finalized, this change in coding policy will result in higher payment rates for hospital patients who are experiencing housing insecurity.
Connect with Us
HMA’s Medicare Practice Group, including consultants from The Moran Company, works to monitor legislative and regulatory developments in the inpatient hospital space and to assess the impact of inpatient payment, quality, and policy changes on the hospital sector. Our Medicare experts interpret and model inpatient policy proposals and use these analyses to assist clients in developing their strategic plans and commenting on proposed regulations. We replicate the methodologies CMS uses in setting hospital payments and model alternative payment policies using the most current Medicare (100%) claims data. We assist clients with modeling for DRG reassignment requests and to support NTAP applications. We also support clients in analyzing CMS Innovation Center alternative payment models.
For more information or questions about the policies described above, contact Zach Gaumer, Amy Bassano, Kevin Kirby or Clare Mamerow.